Research
Research overview
Our group focuses on the preparation and electrocatalytic evaluation of heterogeneous catalysts. High surface area materials with controllable size, facets, and functional groups that are cost-efficient and scalable are of particular importance. We use a systematic approach of first characterizing model surfaces and then transferring them to nanoparticulate systems to create application-oriented materials for use in fuel cells and electrolyzers. The fundamentals of both fields, including surface potential and double layer, are closely interlinked and we use this to gain a deeper understanding of catalytic processes such as the oxygen reduction reaction, oxygen evolution reaction, hydrogen evolution reaction, and carbon dioxide reduction. Our research is supported by fundamental studies on growth mechanisms and the development of new materials. We use innovative, automated characterization techniques to quickly evaluate the performance of numerous materials, which accelerates the discovery of new materials. We also study the corresponding reaction mechanisms essential for understanding the underlying processes.
Synthesis of Nanomaterials
Running (collaborative) projects include:
- Deposition of monolayer thin metals onto non-noble elements to form core-shell structures
- Synthesis of single-atom catalysts for the electrochemical production of hydrogen peroxide
Further reading:
- M. Ledendecker, S. Krick Calderón, C. Papp, H. P. Steinrück, M. Antonietti, M. Shalom, Angew. Chem. Int. Ed. 2015, 54, 12361-12365.
- M. Ledendecker, E. Pizzutilo, G. Malta, G. V. Fortunato, K. J. Mayrhofer, G. J. Hutchings, S. J. Freakley, ACS Catalysis 2020.
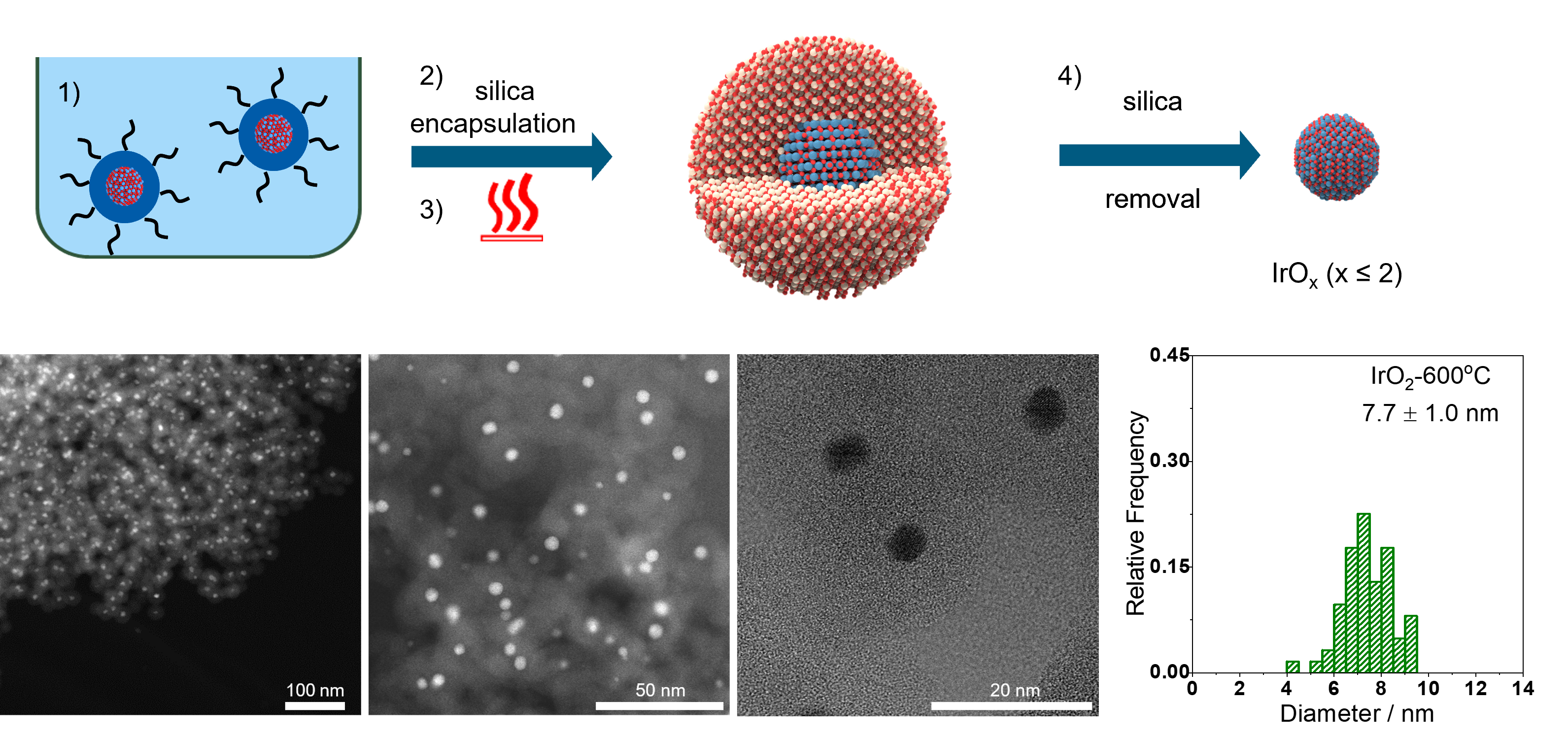
- M. Malinovic, P. Paciok, E. S. Koh, M. Geuss, J. Choi, P. Pfeifer, J. P. Hofmann, D. Goehl, M. Heggen, S. Cherevko, M. Ledendecker, Size-Controlled Synthesis of IrO2 Nanoparticles at High Temperatures for the Oxygen Evolution Reaction, Advanced Energy Materials, 13, 2023
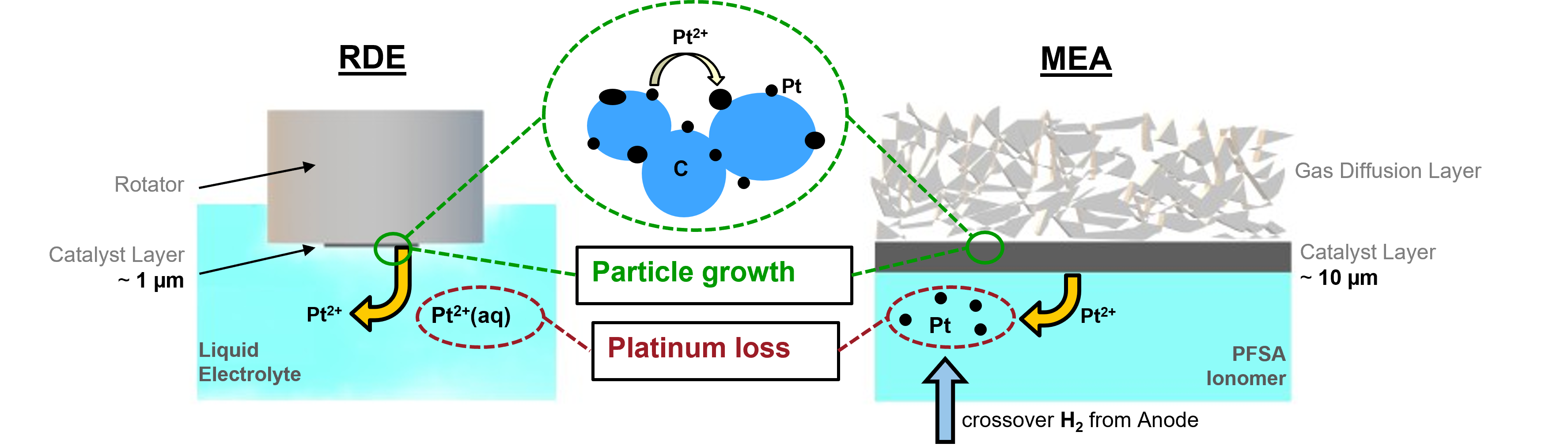
Low-Temperature Fuel Cells
Running (collaborative) projects include:
- Non-carbon-based supports that show sufficient conductivity and can withstand the harsh conditions of the ORR.
- Core-shell nanoparticles and their behavior during ORR.
- Shape-controlled nanoparticles with defined facets for enhanced ORR kinetics.
Further reading:
- D. Göhl, A. Garg, P. Paciok, K. J. Mayrhofer, M. Heggen, Y. Shao-Horn, R. E. Dunin-Borkowski, Y. Román-Leshkov, M. Ledendecker, Nat. Mater. 2020, 19, 287-291.
- D. Jalalpoor, D. Göhl, P. Paciok, M. Heggen, J. Knossalla, I. Radev, V. Peinecke, C. Weidenthaler, K. J. J. Mayrhofer, M. Ledendecker, F. Schüth, J. Electrochem. Soc. 2021, 168, 024502.
- J. Knossalla, P. Paciok, D. Gohl, D. Jalalpoor, E. Pizzutilo, A. M. Mingers, M. Heggen, R. E. Dunin-Borkowski, K. J. J. Mayrhofer, F. Schüth, M. Ledendecker, J. Am. Chem. Soc. 2018, 140, 15684-15689.
Low-temperature electrolyzers
Our research on water electrolysis focuses on the oxygen and hydrogen evolution side, where harsh conditions such as low pH and high potentials (OER) can pose challenges to catalyst stability. One of the main goals of our research is to identify material combinations that can reduce the amount of precious metals needed while maintaining high stability. This is a significant obstacle in this field, and we are working to overcome it by exploring different materials. Our efforts in this area will contribute to the development of more efficient and cost-effective water electrolysis technologies.
- The evaluation of core-shell materials for the oxygen evolution reaction based on IrO2 and non-noble cores
Further reading:
- Patent: Beschichtung und ein Verfahren zum Herstellen von Kern-Schalen-Nanopartikeln, Daniel Göhl, Marc Ledendecker, DE 10 2021 118 226.3, 2021, Technische Universität Darmstadt
- S. Geiger, O. Kasian, M. Ledendecker, E. Pizzutilo, A. M. Mingers, W. T. Fu, O. Diaz-Morales, Z. Li, T. Oellers, L. Fruchter, A. Ludwig, K. J. J. Mayrhofer, M. T. M. Koper, S. Cherevko, Nat. Catal. 2018, 1, 508-515.
- M. Ledendecker, S. Geiger, K. Hengge, J. Lim, S. Cherevko, A. M. Mingers, D. Göhl, G. V. Fortunato, D. Jalalpoor, F. Schüth, C. Scheu, K. J. J. Mayrhofer, Nano Research 2019, 12, 2275-2280.
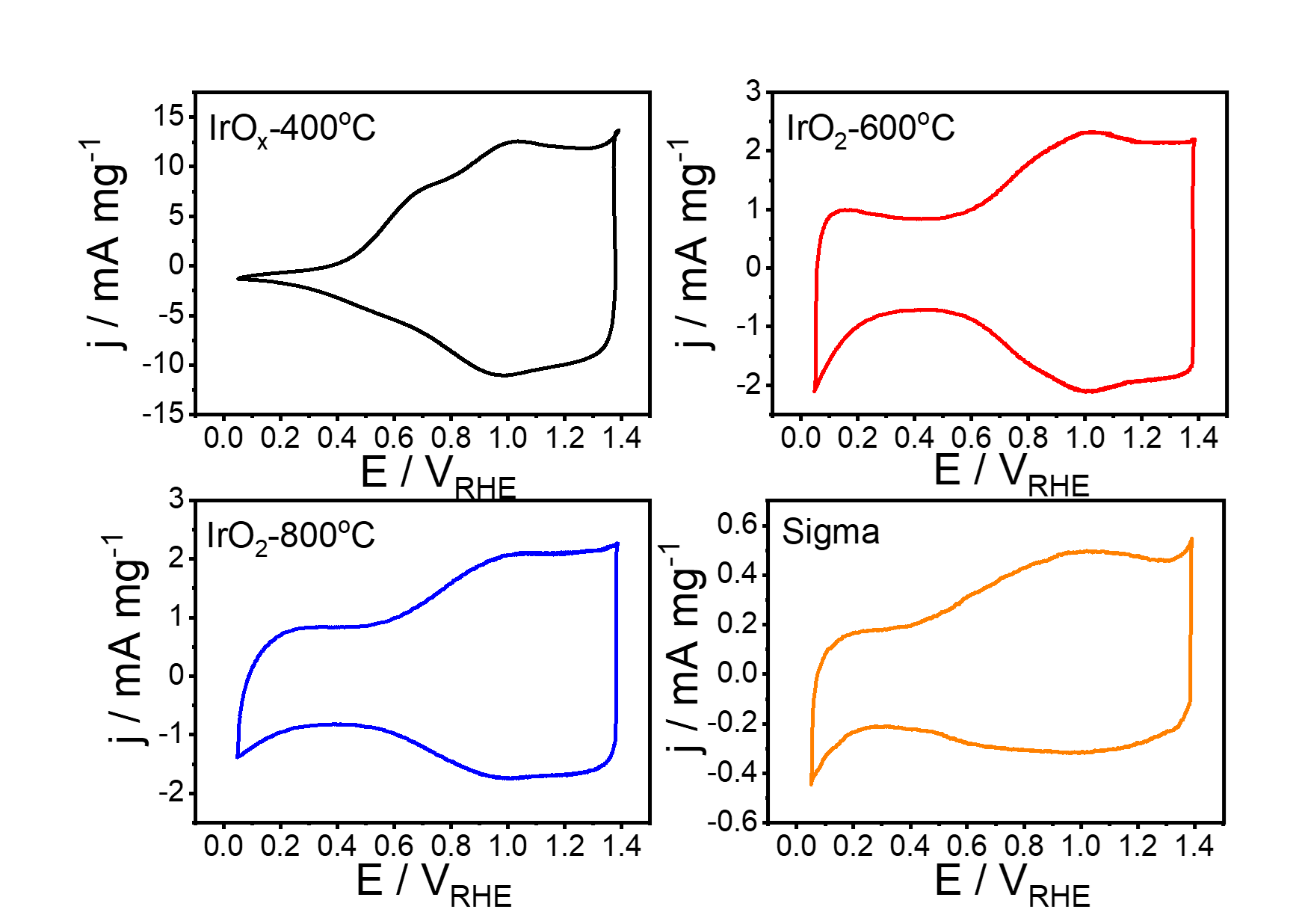
Iridium oxide nanoparticles prepared at different temperatures and their electrochemical response based on the crystallisation degree.
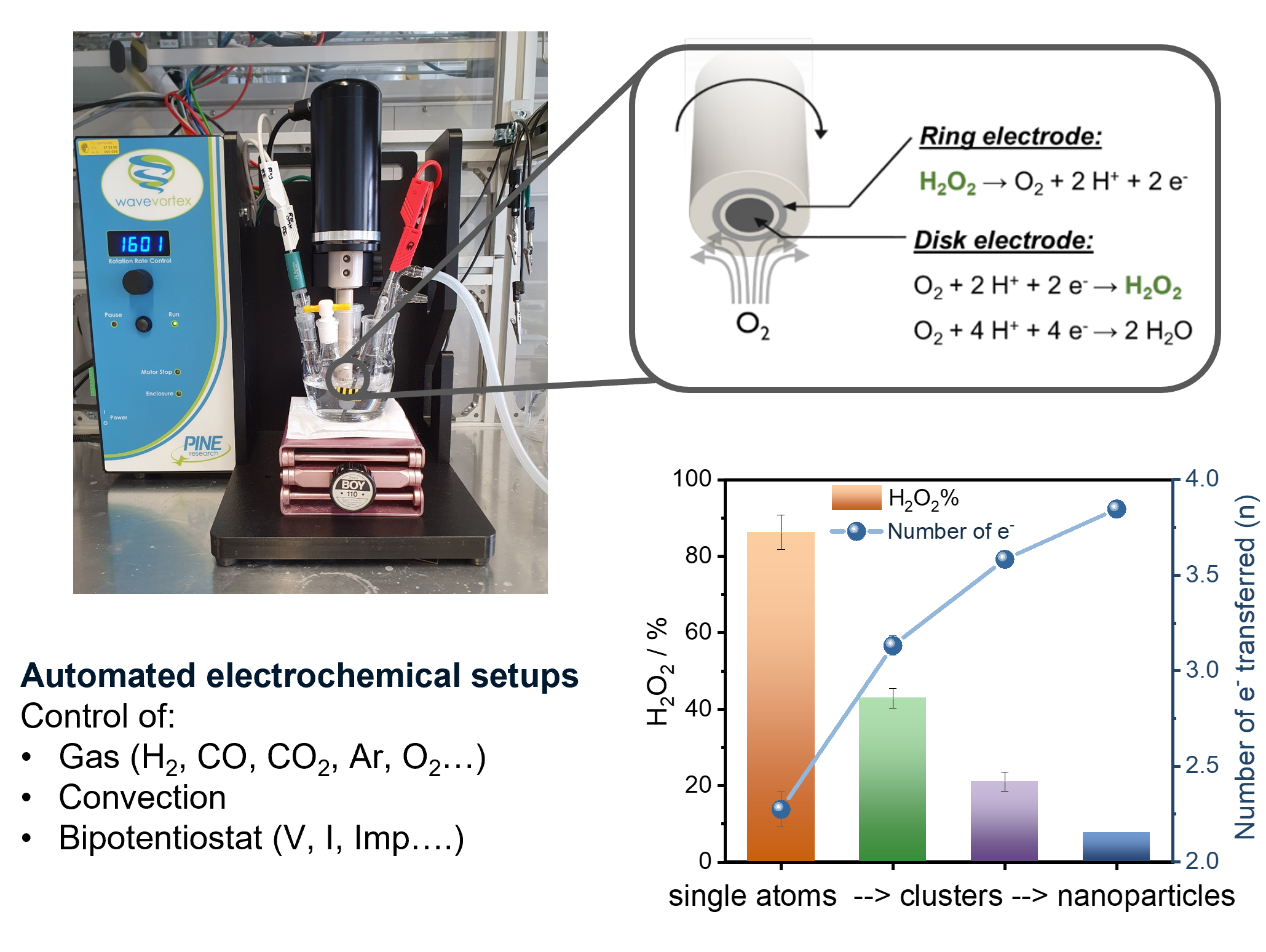
Electrocatalytic H2O2 production
Further reading:
- M. Ledendecker, E. Pizzutilo, G. Malta, G. V. Fortunato, K. J. Mayrhofer, G. J. Hutchings, S. J. Freakley, ACS Catalysis 2020.
CO2 reduction
The long-term stability of catalysts is one of the most important challenges in the electrochemical CO2 reduction (CO2RR). Highly complex and convoluted effects influence the reorganization of copper catalysts during the reaction. Preventing the reorganization of copper catalysts requires an extensive understanding of this process. To this end, our group aims to further understanding these effect by developing novel catalysts and explore the influence of reaction conditions on the stability and selectivity of the electrochemical CO2RR, laying the foundation for the large-scale application of CO2 reduction in the future.
Running (collaborative) projects include:
The influence of pore confinement on the stability of copper catalysts in the electrochemical CO2 reduction.
In-situ studies on the reorganization of copper catalysts in the electrochemical CO2 reduction.
Further readings:
E. S. Koh, S. Geiger, A. Gunnarson, T. Imhof, G. M. Meyer, P. Paciok, B. J. M. Etzold, M. Rose, F. Schueth, M. Ledendecker, Influence of Support Material on the Structural Evolution of Copper during Electrochemical CO2 Reduction, Chemelectrochem, 10, 2023
Method development
We develop new preparation methods for nanoparticle systems, their characterisation and the electrochemical testing. A multitude of self-developed cell designs have been used in the past ranging from classical three electrode setups to flow cells that are coupled to operando analytics
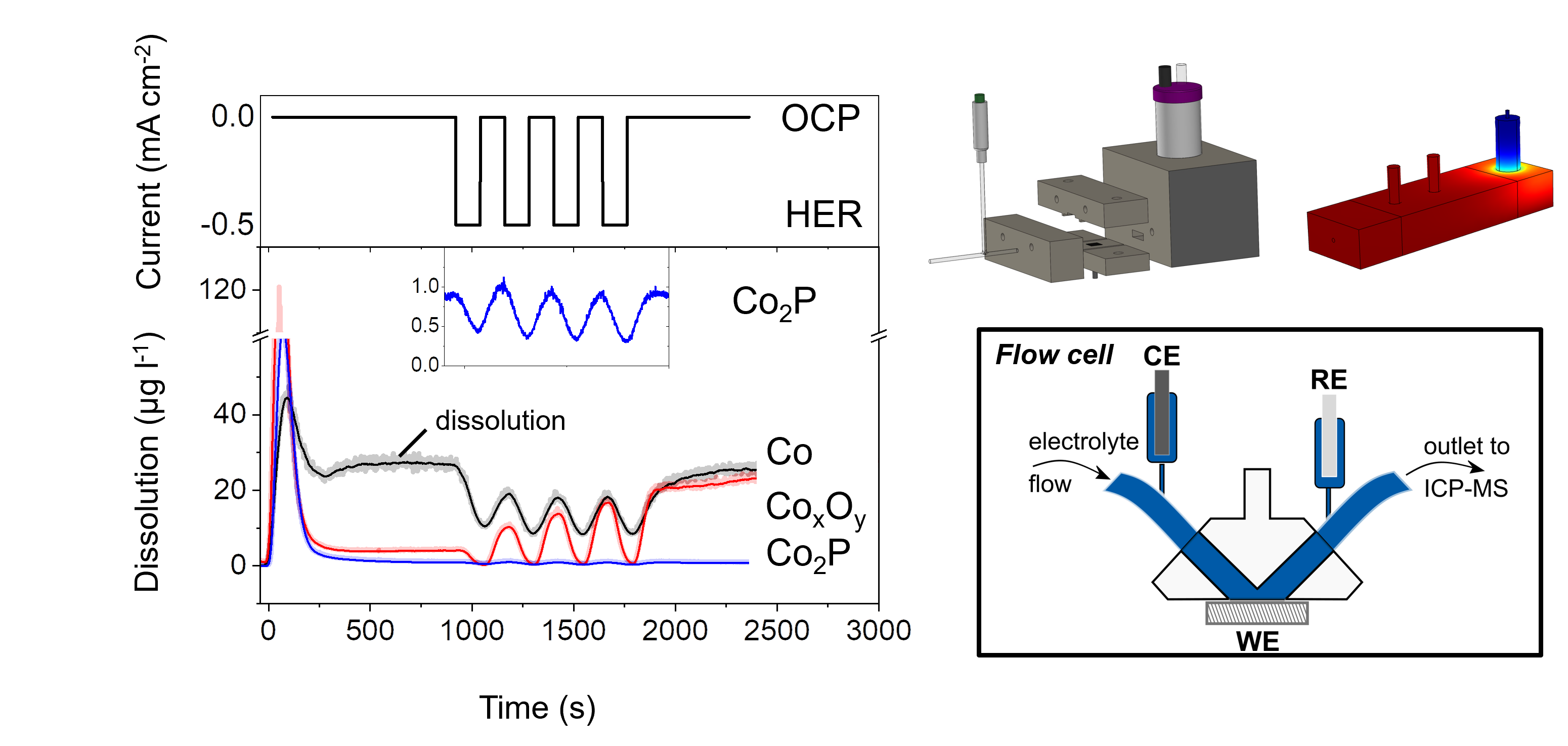
Development of electrochemical flow cells that are coupled to on-line analytics
Ledendecker, J. S. Mondschein, O. Kasian, S. Geiger, D. Göhl, M. Schalenbach, A. Zeradjanin, S. Cherevko, R. E. Schaak, K. Mayrhofer, Angewandte Chemie, 2017
