Research overview
We’re a passionate, curious, and collaborative team working at the interface of materials science, electrochemistry, and catalysis. Our mission: to help shape the future of energy by developing and understanding heterogeneous catalysts for key electrochemical processes—from water splitting to small molecule activation. Our work bridges fundamental surface science and real-world applications, always with an eye on sustainability, scalability, and innovation.
- We thrive on a systematic approach: starting from model surfaces to uncover atomic-scale insights, then translating these to advanced nanoparticulate materials. We push the boundaries of mechanistic understanding with cutting-edge in-situ and ex-situ techniques (yes, we do love our channel flow cells and our ICP-MS setup), and we’re embracing automation to accelerate materials discovery and electrocatalyst testing.
- Based at TUM and the Helmholtz Institute Erlangen-Nürnberg, our group is driven by collaboration, creativity, and a firm belief that energy research can be both rigorous and fun.
Synthesis of Nanomaterials
Maintaining high-performance and long-term stable functional materials requires the ability to control the nanostructure and composition of nanostructured catalysts during synthesis. Therefore, innovative, easily controllable, and scalable synthetic routes for catalyst materials are of great interest. Core-shell materials, for example, combine the properties of a particle core with those of a particle shell, can exhibit synergistic interactions, and also allow for the significant reduction of cost-critical components like precious metals.
Running (collaborative) projects include:
- Deposition of monolayer thin metals onto non-noble elements to form core-shell structures
- Synthesis of single-atom catalysts for the electrochemical production of hydrogen peroxide
Further reading:
- M. Ledendecker, S. Krick Calderón, C. Papp, H. P. Steinrück, M. Antonietti, M. Shalom, Angew. Chem. Int. Ed. 2015, 54, 12361-12365.
- M. Ledendecker, E. Pizzutilo, G. Malta, G. V. Fortunato, K. J. Mayrhofer, G. J. Hutchings, S. J. Freakley, ACS Catalysis 2020.
- M. Malinovic, P. Paciok, E. S. Koh, M. Geuss, J. Choi, P. Pfeifer, J. P. Hofmann, D. Goehl, M. Heggen, S. Cherevko, M. Ledendecker, Size-Controlled Synthesis of IrO2 Nanoparticles at High Temperatures for the Oxygen Evolution Reaction, Advanced Energy Materials, 13, 2023
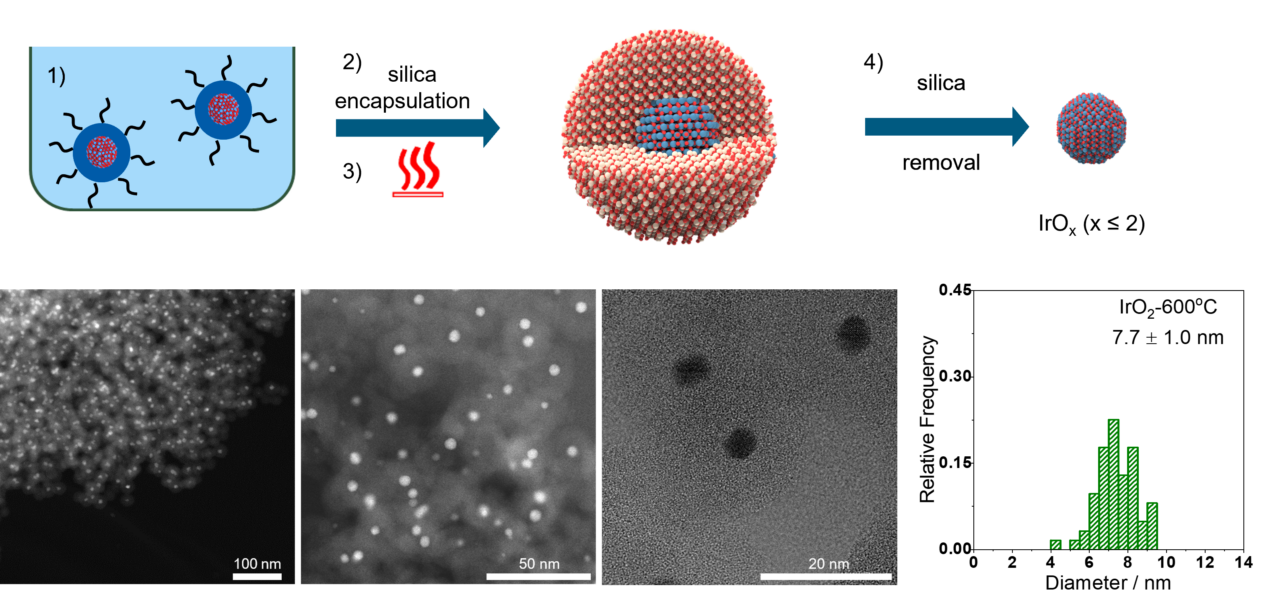
Low-temperature Fuel Cells
In our research on low temperature fuel cell catalysts, we focus on materials for proton exchange membrane (PEM) fuel cells. One of the main challenges in this field is finding materials that are stable enough for use in PEM fuel cells, particularly non-noble and noble metals. To address this challenge, we use innovative, automated characterization techniques to evaluate the performance of different materials as catalysts. This allows us to quickly examine a large number of materials and accelerate the discovery of new, stable materials. We also determine structure-performance parameters, which are crucial for understanding the processes that occur during catalysis and how they affect fuel cell performance.
Running (collaborative) projects include:
- Non-carbon-based supports that show sufficient conductivity and can withstand the harsh conditions of the ORR.
- Core-shell nanoparticles and their behavior during ORR.
- Shape-controlled nanoparticles with defined facets for enhanced ORR kinetics.
Further reading:
- D. Göhl, A. Garg, P. Paciok, K. J. Mayrhofer, M. Heggen, Y. Shao-Horn, R. E. Dunin-Borkowski, Y. Román-Leshkov, M. Ledendecker, Nat. Mater. 2020, 19, 287-291.
- D. Jalalpoor, D. Göhl, P. Paciok, M. Heggen, J. Knossalla, I. Radev, V. Peinecke, C. Weidenthaler, K. J. J. Mayrhofer, M. Ledendecker, F. Schüth, J. Electrochem. Soc. 2021, 168, 024502.
- J. Knossalla, P. Paciok, D. Gohl, D. Jalalpoor, E. Pizzutilo, A. M. Mingers, M. Heggen, R. E. Dunin-Borkowski, K. J. J. Mayrhofer, F. Schüth, M. Ledendecker, J. Am. Chem. Soc. 2018, 140, 15684-15689.
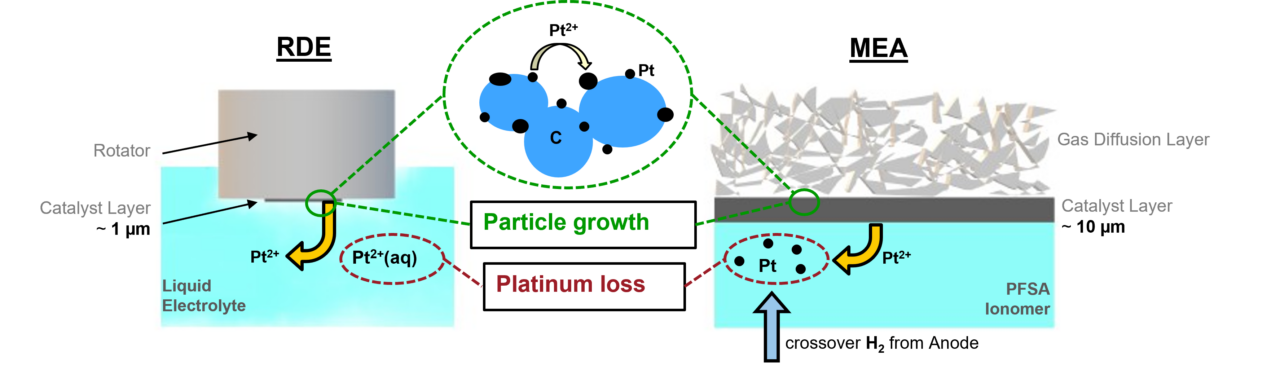
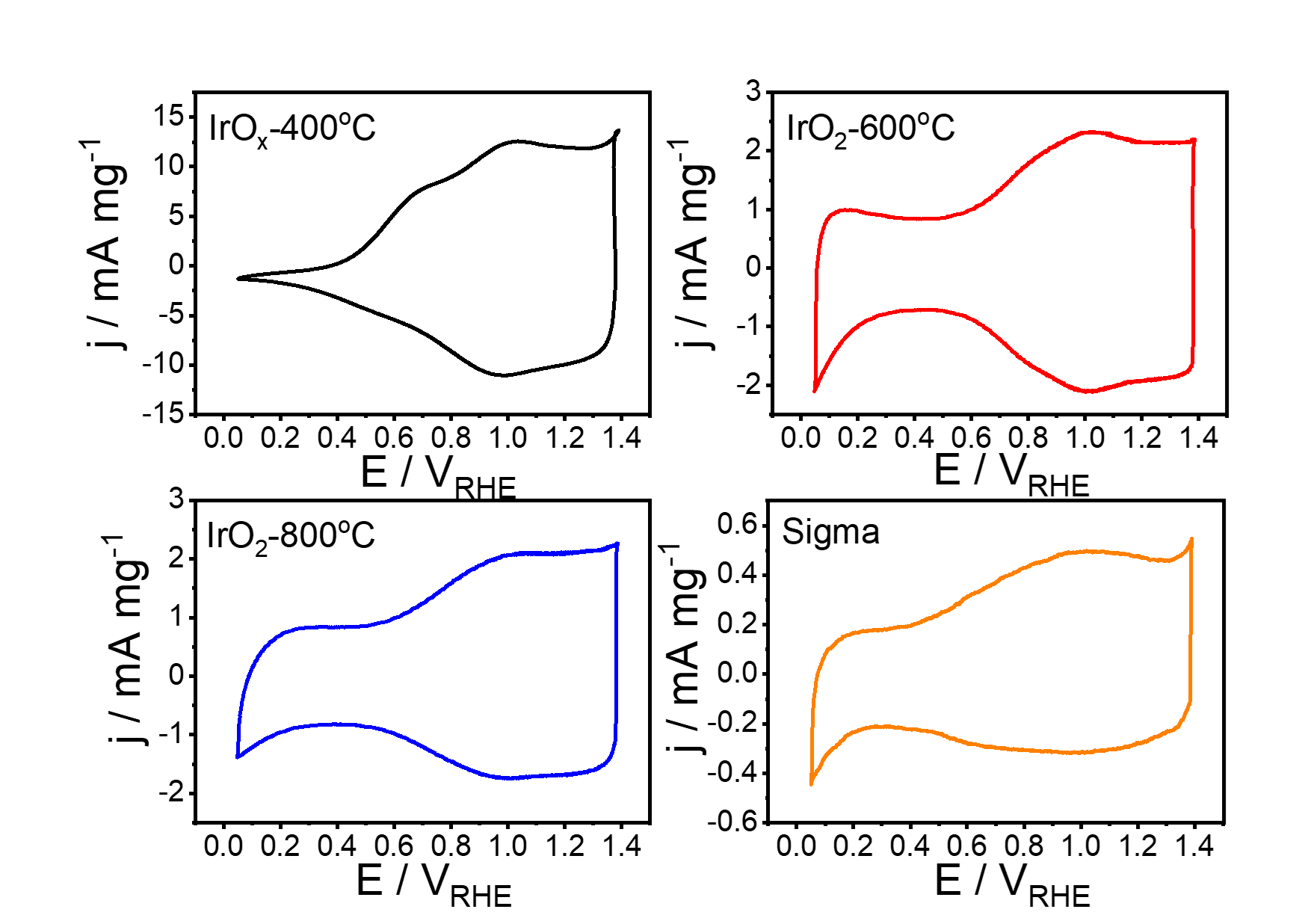
Low-temperature electrolyzers
Our research on water electrolysis focuses on the oxygen and hydrogen evolution side, where harsh conditions such as low pH and high potentials (OER) can pose challenges to catalyst stability. One of the main goals of our research is to identify material combinations that can reduce the amount of precious metals needed while maintaining high stability. This is a significant obstacle in this field, and we are working to overcome it by exploring different materials. Our efforts in this area will contribute to the development of more efficient and cost-effective water electrolysis technologies.
Running (collaborative) projects include:
- The evaluation of core-shell materials for the oxygen evolution reaction based on IrO2 and non-noble cores
Further reading:
- Patent: Beschichtung und ein Verfahren zum Herstellen von Kern-Schalen-Nanopartikeln, Daniel Göhl, Marc Ledendecker, DE 10 2021 118 226.3, 2021, Technische Universität Darmstadt
- S. Geiger, O. Kasian, M. Ledendecker, E. Pizzutilo, A. M. Mingers, W. T. Fu, O. Diaz-Morales, Z. Li, T. Oellers, L. Fruchter, A. Ludwig, K. J. J. Mayrhofer, M. T. M. Koper, S. Cherevko, Nat. Catal. 2018, 1, 508-515.
- M. Ledendecker, S. Geiger, K. Hengge, J. Lim, S. Cherevko, A. M. Mingers, D. Göhl, G. V. Fortunato, D. Jalalpoor, F. Schüth, C. Scheu, K. J. J. Mayrhofer, Nano Research 2019, 12, 2275-2280.
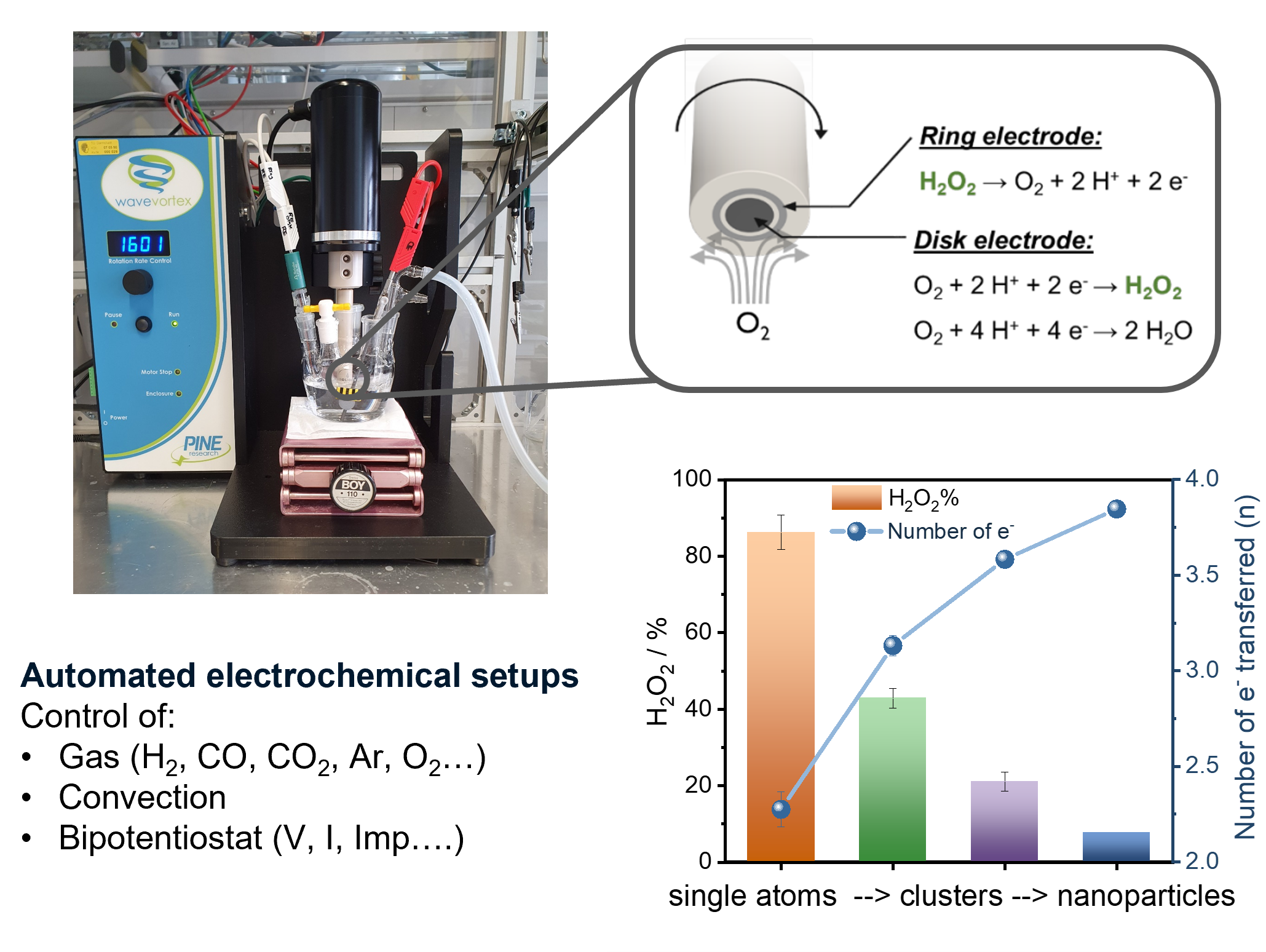
Electrocatalytic H2O2 production
Our group works actively on the electrocatalytic reduction of oxygen to H2O2. In classical heterogeneous catalysis, the selective production H2O2 from molecular hydrogen and oxygen has been a target reaction since the beginning of the 20th century. Through electrochemical half-cell testing, both reactions can be investigated independently and conclusions for the heterogeneous synthesis of H2O2 can be drawn. Single atoms of palladium supported on high surface area carbon supports have demonstrated to be highly selective and active towards the production of H2O2. Here, we rely on strong collaboration partners from the field of classical heterogeneous catalysis (Simon Freakley, Graham Hutchings, Karl Mayrhofer and Ioannis Katsounaros).
Further reading:
- M. Ledendecker, E. Pizzutilo, G. Malta, G. V. Fortunato, K. J. Mayrhofer, G. J. Hutchings, S. J. Freakley, ACS Catalysis 2020.
CO2 reduction
The long-term stability of catalysts is one of the most important challenges in the CO2 reduction (CO2RR). Highly complex and convoluted effects influence the reorganization of copper catalysts during the reaction. Preventing the reorganization of copper catalysts requires an extensive understanding of this process. To this end, our group aims to further understanding these effect by developing novel catalysts and explore the influence of reaction conditions on the stability and selectivity of the electrochemical CO2RR, laying the foundation for the large-scale application of CO2 reduction in the future.
Running (collaborative) projects include:
The influence of pore confinement on the stability of copper catalysts in the electrochemical CO2.
In-situ studies on the reorganization of copper catalysts in the electrochemical CO2 reduction.
Further readings:
E. S. Koh, S. Geiger, A. Gunnarson, T. Imhof, G. M. Meyer, P. Paciok, B. J. M. Etzold, M. Rose, F. Schueth, M. Ledendecker, Influence of Support Material on the Structural Evolution of Copper during Electrochemical CO2 Reduction, Chemelectrochem, 10, 2023
Method development
We develop new preparation methods for nanoparticle systems, their characterisation and the electrochemical testing. A multitude of self-developed cell designs have been used in the past ranging from classical three electrode setups to flow cells that are coupled to operando analytics